
Clinical Trial Sourcing
DRS Suportemed simplifies de supply of medicines, comparators and supplies for clinical trials, serving globally and in compliance with ANVISA, FDA and EMA regulations.
Contact Us

Unified management of the entire supply chain with global reach
Through direct access to the industry and global strategic partners, we are prepared to meet the specific requirements of your project, such as batches, deadlines, and specific documentation. In an integrated manner, we provide equipment, supplies, materials, and laboratory kits to meet the project's needs.
DRS SUPORTEMED
Clinical research projects in the pharmaceutical industry require a high level of planning and the management of various suppliers involved in different stages, from the supply of raw materials to the distribution of medications and supplies.
Global Networking
Comprising partners in Brazil and around the world, we are qualified to find the best supply options for your projects.
Specialized Company
Experience in providing supplies and specialized logistic management processes for clinical studies.
Importation
All import processes in compliance with global regulations such as ANVISA, FDA, and EMA.
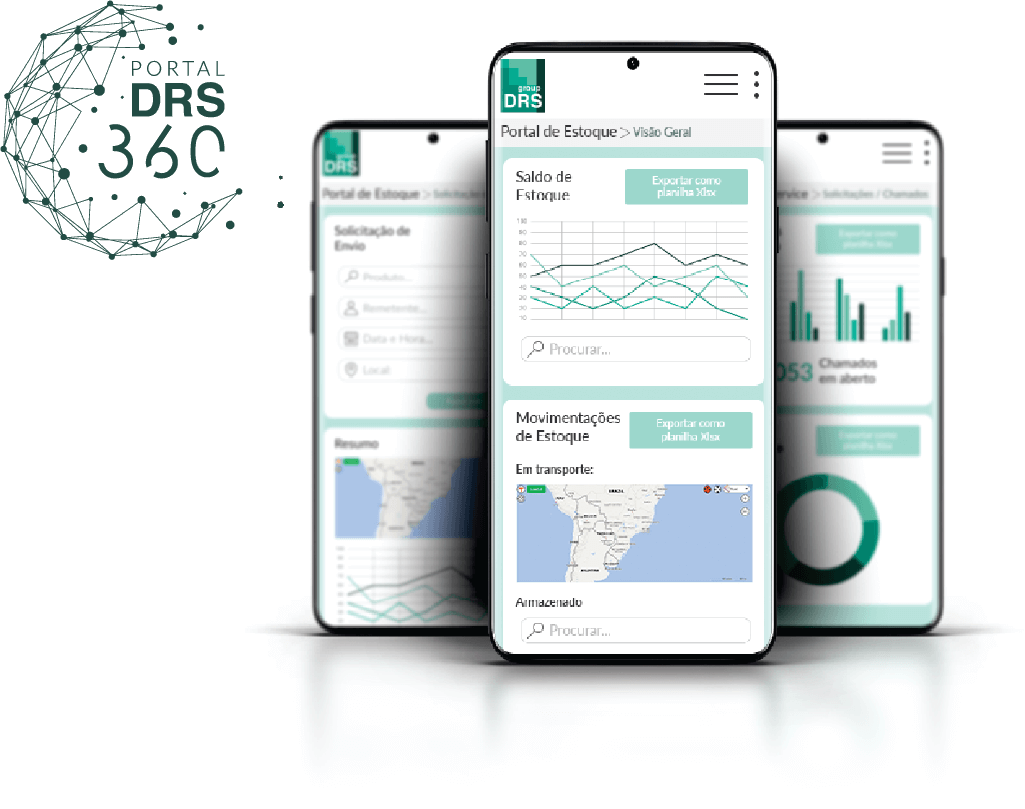
Digital management through DRS 360 portal
All services integrated in a single platform, all project-relevant information at your fingertips

Global supply of medication, equipment and supplies for clinical trials and patient support projects.
Learn More >
Import and export of medicines, supplies, GMOs, and biological samples for healthcare projects.
Learn More >
Global transportation of products and samples that require temperature control and specialized handling for direct-to-patient care.
Learn More >
Technological storage at all temperature ranges and drug classes, including GMOs and biological samples.
Learn More >
Integrated care with specialized logistic services, enriching the personalized patient journey and ensuring their well-being.
Learn More >